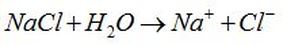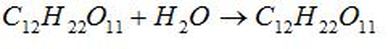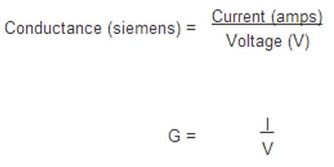Electrolytes
An electrolyte is a substance that produces ions when dissolved in a solvent (usually water). Electrolytes are generally present in the human body as minerals in the blood and other body fluids that carry an electric charge. They can affect the amount of water in the body, the acidity of blood (pH), muscle function, and other important processes. Electrolytes are lost when the body sweats since electrolytes are present in sweat. This is the reason that athletes need to replenish their electrolytes regularly.
How are electrolytes formed?
Electrolyte solutions are generally made when a salt (like table salt/NaCl) is placed into a solvent (a substance able to dissolve other substances) such as water and the individual components dissociate due to the thermodynamic interactions between solvent and solute molecules, this process is called solvation.
When table salt is placed in water the salt dissolves into its component ions:
An electrolyte is a substance that produces ions when dissolved in a solvent (usually water). Electrolytes are generally present in the human body as minerals in the blood and other body fluids that carry an electric charge. They can affect the amount of water in the body, the acidity of blood (pH), muscle function, and other important processes. Electrolytes are lost when the body sweats since electrolytes are present in sweat. This is the reason that athletes need to replenish their electrolytes regularly.
How are electrolytes formed?
Electrolyte solutions are generally made when a salt (like table salt/NaCl) is placed into a solvent (a substance able to dissolve other substances) such as water and the individual components dissociate due to the thermodynamic interactions between solvent and solute molecules, this process is called solvation.
When table salt is placed in water the salt dissolves into its component ions:
Strong electrolytes are substances that only exist as ions in solution. They completely dissociate to their ions when dissolved in solution.
A weak electrolyte only partially dissociates in solution and produces relatively few ions (exist in water as a mixture of individual ions as well as intact molecules).
A non-electrolyte does not dissociate at all (present entirely as intact molecules) in solution and therefore does not produce any ions. Non-electrolytes do dissolve in water as molecules instead of ions. They do not conduct electricity at all.
Example: Sugar
A weak electrolyte only partially dissociates in solution and produces relatively few ions (exist in water as a mixture of individual ions as well as intact molecules).
A non-electrolyte does not dissociate at all (present entirely as intact molecules) in solution and therefore does not produce any ions. Non-electrolytes do dissolve in water as molecules instead of ions. They do not conduct electricity at all.
Example: Sugar
What is the best method of gaining electrolytes?
Most athletes turn to drink “sports drinks” in order to gain electrolytes. These drinks advertise that they contain electrolytes e.g. Powerade says it has an “Advanced electrolytes system” on its label. It is also possible to gain electrolytes by eating table salt but this causes various unhealthy side effects. Many foods also offer electrolytes but they are hard to eat on the go/at a race. Milk also contains electrolytes. Another way to gain electrolytes is by drinking fruit juices.
Most athletes turn to drink “sports drinks” in order to gain electrolytes. These drinks advertise that they contain electrolytes e.g. Powerade says it has an “Advanced electrolytes system” on its label. It is also possible to gain electrolytes by eating table salt but this causes various unhealthy side effects. Many foods also offer electrolytes but they are hard to eat on the go/at a race. Milk also contains electrolytes. Another way to gain electrolytes is by drinking fruit juices.
Major Electrolytes
· Sodium (Na+)
· Potassium (K+)
· Chloride (Cl-)
· Calcium (Ca2+)
· Magnesium (Mg2+)
· Bicarbonate (HCO3-)
· Phosphate (PO42-)
· Sulphate (SO42-)
· Sodium (Na+)
· Potassium (K+)
· Chloride (Cl-)
· Calcium (Ca2+)
· Magnesium (Mg2+)
· Bicarbonate (HCO3-)
· Phosphate (PO42-)
· Sulphate (SO42-)
Electrical Current (Amps)
Current is the flow of electricity which is caused by the ordered directional movement of electrically charged particles. Current is measured in amperes. In this experiment we use a multimeter to measure the amps (specifically milliamps) in our circuit.
Current is the flow of electricity which is caused by the ordered directional movement of electrically charged particles. Current is measured in amperes. In this experiment we use a multimeter to measure the amps (specifically milliamps) in our circuit.
Voltage (Volts)
Voltage is essentially a way to describe an object’s electric field using numbers. An object’s electric field is quite like a magnetic field and shares some properties like being invisible or the ability to attract and repel objects. Voltage is what causes “static electricity” due to its voltage fields which attract or repel objects. In other words, voltage is an electromotive force or potential difference expressed in volts. In this experiment we use a 9V battery to power the circuit.
Electrical conductance (siemens)
Electrical conductance is an expression of the ease with which an electrical current passes through a substance (in this experiment the juices). Electrical conductance is measured in siemens. Our final aim in this experiment was to calculate the conductance of each liquid. This was achieved by using the following formula:
Voltage is essentially a way to describe an object’s electric field using numbers. An object’s electric field is quite like a magnetic field and shares some properties like being invisible or the ability to attract and repel objects. Voltage is what causes “static electricity” due to its voltage fields which attract or repel objects. In other words, voltage is an electromotive force or potential difference expressed in volts. In this experiment we use a 9V battery to power the circuit.
Electrical conductance (siemens)
Electrical conductance is an expression of the ease with which an electrical current passes through a substance (in this experiment the juices). Electrical conductance is measured in siemens. Our final aim in this experiment was to calculate the conductance of each liquid. This was achieved by using the following formula: